Fagus grandifolia Ehrh. - American Beech, Beechnut
Common Names
American Beech, BeechnutField Identification
A medium to large tree with simple, alternate small-toothed leaves which usually wither but remain attached in winter; bark usually smooth and silvery-gray and tan buds long-cylindrical and pointed.Food uses
Disclaimer: The information provided here is for reference and historical use. We do not recommend nor do we condone the use of this species for food purposes without first consulting a physician.
(Moerman, 1998) Nuts eaten by Native Americans and utilized to make drinks, breads, cakes, puddings, pies, gravies, and to season other foods.
Medicinal uses
Disclaimer: The information provided here is for reference and historical use. We do not recommend nor do we condone the use of this species for medicinal purposes without first consulting a physician.
(Moerman, 1998) Used by Native Americans to treat worms, frostbite, rashes, burns and other skin maladies, tuberculosis, venereal disease, and as a blood purifier.
Other uses
Wood used for boxes and crates, flooring, interior finishes, tool handles, woodenware, railroad ties and also for fuel, charcoal, and wood-distillation purposes. (Hill, 1952)Used by Native Americans to make bowls, buttons, snowshoe frames and general building material. Also the nutmeat oil was mixed with bear grease and used to ward off mosquitoes. (Moerman, 1998)
Stories
Beech [the European species] was one of the first trees to be used for writing material. The Anglo-Saxon word for beech was “boc”, which became the English word "book".
Nomenclature
Fagus grandifolia Ehrhart, Beitr. Naturk. 3: 23. 1783.Fagus americana latifolia Munchh., Hausvat. 5: 162. 1770.
Fagus sylvatica c. Americana latifolia Du Roi, Harbk. Baumz. 1: 269. 1771.
Fagus Americana Lodd. ex C. F. Ludwig, Neu. Wilde Baumz. 19. 1783.
?Fagus sylvatica var. latifolia C. F. Ludwig, Neu. Wilde Baumz. 19. 1783.
Fagus sylvatica atropunicea Marsh., Arbust. Am. 46. 1785. not Weston 1770.
Fagus ferruginea Ait., Hort. Kew. 3: 362. 1789.
Fagus alba Raf., Fl. Ludovic. 131. 1817.
Fagus sylvatica var. americana Nutt., Gen. N. Am. Pl. 2: 216. 1818.
Fagus americana Sweet, Hort. Brit. 370. 1826.
Fagus heterophylla Raf., New Fl. N. Am. 3: 80. 1838.
Fagus ferruginea var. latifolia Loudon, Arb. Brit. 3: 1918, fig. 1916. 1838.
Fagus castanaefolia C. de Vos, Handb. Boom. Heest. ed. 2, 72. 1887.
Fagus atropunicea (Marsh.) Sudworth in Bull. Torrey Bot. Club, 20: 42. 1893.
Fagus latifolia (Milnchh.) Sudworth in U. S. Dept. Agric. For. Serv. Bull. 14: 148. (Nomencl. Arb. Fl. U. S.) 1897.
Fagus grandifolia var. caroliniana Fernald & Rehder, Rhodora 9: 114. 1907.
Fagus grandifolia var. typica Rehd., in Mitt. Deutsch. Dendr. Ges. 1907(16): 70. 1908.
TYPE: unknown
For a synopsis of the proposed existence of infraspecific taxa see Nixon (p. 444), 1997.
Recent research on Fagus grandifolia population genetics using allozyme polymorphisms indicate two genetically distinct clusters, one from the Gulf-coastal plain, eastern coastal plain, Piedmont Plateau and Ozark Plateau; and the other from the remaining northern glaciated territories (Kitamura & Kawano, 2001).
For recent research of variation in cupule appendages between northern and southern populations see Denk & Miller, 2001.
Description
HABIT Perennial, deciduous (usually marcescent), phanerophytic, tree, diclinous and monoecious, 20-30 m tall. For a detailed study of the architecture and development pattern see Millet et al., 1998.STEMS Main stems ascending to erect, round. Bark typically smooth, light gray to gray, not exfoliating (occasionally curly- barked or fissured-bark patterns are found, for a review these anomalous bark types, and the micro-anatomy and variation of the periderm see Ostrofsky & Blanchard, 1984 and (Rice, 1948)). Branches erect or ascending or horizontal. Twigs brown to reddish-brown to gray, terete, 2-5 mm in diameter, often zig-zag, smooth or lenticellate, 2nd year and older glabrous, 1st year with long and unbranched erect to spreading light brown hairs, sparsely distributed, glabrescent. Pith white, round, small, continuous; nodal diaphragm absent. Sap translucent. For a study of xylem vessel length see Zimmermann & Jeje, 1981. Roots can form suckers with an effective range of 10-15 m (Kitamura et al., 2000) and may be the main mode of reproduction in certain areas where the habitat parameters are more severe (Held, 1983) (Morris, et al., 2004). For an investigation on the micro-anatomy of the root sprout initiation zone see Jones & Raynal, 1986. For a study of unique regional patterns of differentiation in genetic components among populations with and without vegetative regeneration by root suckers see Kitamura & Kawano, 2001. For a review of the micro-anatomy of the root/mycorrhizal interface see Vozzo & Hacskaylo, 1964.
BUDS Pseudo-terminal and axillary present (often mostly pseudo-terminal), scattered along stem. Pseudo-terminal bud fusiform, 12-20 mm long, pointed; axillary buds 1 per axil, fusiform, 12-20 mm long, pointed. Bud scales 10+, usually tan, sometimes dark brown to brown, imbricate, chartaceous, glabrous or with short and unbranched appressed, brown or light brown or light gray hairs, sparsely distributed apically, not glabrescent. Bud scale scars encircling the twig. Leaf scars half-round. Vascular bundle scars 3, or multiple. Stipule scars linear, nearly meeting around the twig.
LEAVES Alternate, simple, (first leaves of seedling are opposite and decussate in relation to cotyledons, afterwards leaves alternate and distichous (Millet et al., 1998)), spaced somewhat evenly along and divergent from stem. Stipules lateral, free from the petiole, narrow, 2-4 cm long, caducous. Leaves petiolate, petiole proximally terete; distally grooved adaxially, 0.3-0.8 cm long, with long and unbranched light -brown erect to spreading hairs, sparsely to moderately dense, distributed throughout; not glabrescent. Leaf blades: abaxial surface light green, adaxial surface green to bluish-green, elliptic or ovate or obovate, bilaterally symmetric, 5-18 cm long, 3-9 cm wide, chartaceous, base cuneate (occasionally minutely sub-cordate or oblique), margin serrate with white hairs, apex acuminate or acute; pinnately veined. Abaxial surface with long and unbranched erect to spreading white hairs, sparsely distributed along midveins and secondary veins. Adaxial surface with long and unbranched erect to spreading white hairs, sparsely distributed along midveins and secondary veins. In addition to the aforementioned visible hairs, 3 other minute hair types (40+ x magnification needed) have been described on the leaf surfaces: uni- or multi-cellular simple, multi-cellular bulbous, and uni-cellular acicular. For a study of micro-morphology and variation of these hairs and the leaf cuticle, and the ecotypic and geographic variation in vestiture see Hardin & Johnson, 1985. For an investigation of the epidermal micro-anatomy see Denk, 2003. For an investigation of the mesophyll micro-anatomy see Dengler & MacKay, 1975 and Dengler et al., 1975.
FEMALE INFLORESCENCES Coetaneous cupule containing 2 flowers, axillary in new growth of leaves. Cupule persistent, composed of numerous awn- shaped appendages ("bracts"), the outer often bright red and remaining free and becoming indurate-spiny at maturity, the inner united to form a 4-valved (valves 7-18 mm long, narrowly triangular to ovate to suborbicular; for a study of variation in cupule appendage morphology between northern and southern populations see Denk & Miller, 2001)) covering of the fruits; densely covered with short brown hairs. There has been debate over the years regarding the true ontogenetic nature of the cupule. Originally thought to be an involucre of bracts, recent research suggests that the cupule is a complex partial inflorescence derived from stem tissue, see Abbe, 1974;Brett, 1964;Foreman, 1966;MacDonald, 1979;Fey & Endress, 1983. Peduncle .8-2.5 cm long, moderately covered with light-brown sericeous hairs. True bracts sessile, linear, apex acute, caducous. For a new interpretation that the inflorescence is indeterminate see Okamoto,1989. For an ontogenetic and anatomical study see Langdon, 1939;Garrison, 1957.
FEMALE FLOWERS Perianth of one whorl, calyx urceolate, of fused sepals, fragrance absent. Gynoecium syncarpous. Carpels 3. Locules 3, each containing 2 ovules. Styles 3. Ovary inferior. Placentation axile. Occasionally rudimentary stamens attached to the top of the style (Langdon, 1939). For an ontogenetic and anatomical study see Langdon, 1939;Garrison, 1957.
MALE INFLORESCENCES Coetaneous, capitulum (dichasia) of 7-25, (average 19) (Rebuck, 1952) flowers, axillary in new growth of leaves. Peduncle 2-4.5 cm long, moderately covered with light-brown sericeous hairs. Bracts sessile, linear, apex acute, caducous. For a new interpretation that the inflorescence is indeterminate see Okamoto, 1989. For an ontogenetic and anatomical study see Langdon, 1939;Garrison, 1957.
MALE FLOWERS Perianth of one whorl, 4-6(8) lobed, campanulate, moderately covered with light brown sericeous hairs; pedicles 1-2.5 mm, moderately covered with light brown sericeous hairs; fragrance absent. Stamens 6-12 (16), exserted, adnate at base of perianth. Anthers, light yellow, glabrous, basifixed, opening along the long axis. Filaments free, straight, white, glabrous. For an ontogenetic and anatomical study see Garrison, 1957.
FRUITS Cupule indurate, 4-valved, brown, ovoid to subglobose, 15-20 mm long, 15-20 mm wide, with stout prickles, and with short and unbranched brown hairs densely distributed throughout; containing 2 (rarely 3?) trymosa (Spjut, 1994). Trymosum brown, lustrous, ovoid, unequally triangular, prominently winged to slightly winged on angles, rostrate, one seeded by abortion of the other 5 ovules. Maturing in one season. For an ontogenetic and anatomical study see Langdon, 1939;Garrison, 1957.
SEEDS Seeds up to 15 mm long, broadly ovoid, dark brown, glabrous, lacking endosperm. For an ontogenetic and anatomical study see Garrison, 1957. Average weight 3.5 grams (Young & Young, 1992).
Habitat
Usually mesic rich woods, often in deep shade; occasionally found in semi-hydric forest sites or even xeric thin soils of the Pine Barrens.Distribution
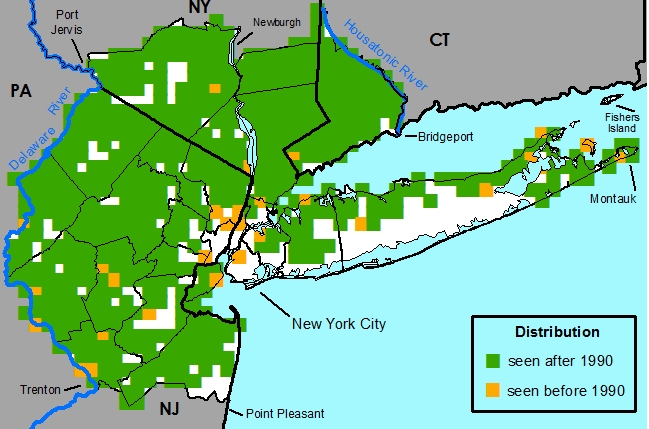
Indigenous to eastern North America.United States -- AL, AR, CT, DE, FL, GA, IL, IN, KY, LA, MA, MD, ME, MI, MO, MS, NC, NH, NJ, NY, OH, OK, PA, RI, SC, TN, TX, VA, VT, WI, WV
Canada -- NB, NS, ON, PE, QC
New York Metropolitan Region -- Native throughout the metropolitan region.
Rarity Status
Rarity Status
Global Heritage Rank -- G5
Connecticut -- Not listed
New Jersey -- Not listed
New York -- Not listed
Species Biology
Flowering (April [week 1]) April [week 3] - May [week 4] (June [week 2])Pollination Anemophily
Fruiting June [week 2] - September [week 4]
Known for masting, one study found a close relationship between masting and preceding growing season climate events. A drought in the early summer preceding masting (mast year-1) is a very strong predictor which is increased if there has been an unusually moist, cool summer the year before the drought (mast year-2). It is hypothesized that, in this initial moist summer (mast year-2), carbohydrate buildup within the trees primes them for floral induction the following year (year-1). A drought event in the early part of the following summer (mast year-1) acts as a trigger for the release of those reserves into flower initiation and then seed production. (Piovesan & Adams, 2001)
Sometimes producing crops of seedless fruits (Garrison, 1957) .
Dispersal Probably small predators of nuts facilitate dispersal by dropping undamaged nuts and failing to recover cached nuts; these include Sciurus carolinensis (gray squirrel), Sciurus niger (fox squirrel), Glaucomys volans (southern flying squirrel), Tamias striatus (eastern chipmunk), Peromyscus leucopus (white-footed mouse), Peromyscus maniculatus (deer mouse) and Quiscalus quiscula (common grackle) and Cyanocita cristata (blue jay). (Johnson & Adkisson, 1985)) (Schopmeyer, 1974)
Germination (Young & Young, 1992) (Schopmeyer, 1974)
In nature, germinating in early spring to early summer of the following year; epigeal. One study found seed viability varied considerably from different sites, ranging from 0-93% (Stalter, 1982).
Fall sowing or stratification in moist sand at 41dF has given good results. For long term storage, nuts should be dried to 8 to 10% moisture and stored in sealed containers at -15dC; nuts for spring planting should be dried to 20-30% moisture and stored in sealed containers at 2 to 5dC.