Quercus montana Willd. - Chestnut Oak, Rock Oak, Rock C
Common Names
Chestnut Oak, Rock Oak, Rock CField Identification
Medium-sized to large tree with alternate, simple, toothed leaves; bark of mature tree with large ridges and grooves; buds clustered near twig ends; catkin-like flowers in spring followed by acorns in the fall.Food uses
Disclaimer: The information provided here is for reference and historical use. We do not recommend nor do we condone the use of this species for food purposes without first consulting a physician.
Native American often used the acorns to make various food products, usually by removing the tannins by boiling the nuts or soaking them in lye water. (Moerman, 1998)
Other uses
Wood prized for cabinet work, flooring, finish carpentry, and barrels for beer, wine, and other alcoholic spirits. (Hill, 1952)Oak-mast surveys may help wildlife agencies better understand dynamics of fall harvests and may be useful in harvest management models that attempt to stabilize fall harvest rates of game animals (Norman, 2003).
Tannins derived from oaks have been used historically to tan animal hides into leather. (Burrows & Tyrl, 2001)
Used to make railroad ties.(Moerman, 1998)
Native Americans used the bark to make a tan dye. (Moerman, 1998)
Poisonous Properties
Oak leaves, buds, bark, and acorns contain tannins which have varying degrees of toxicity in different animals. Although oak foliage and acorns provide valuable food for many wildlife species and even some livestock, oak toxicosis, a urinary and digestive tract disease can occur when some animals are forced to subsist on oaks exclusively for several days. Poisoning is rare in humans due to the large amounts needed to ingest to cause symptoms. (Burrows & Tyrl, 2001)
Nomenclature
Quercus montana Willd., Sp. Pl. 4(1): 440. 1805. TYPE: unknown*Quercus prinus L., Sp. Pl. 995. 1753. p.p. nomen ambiguum, see Whittemore & Nixon, 2005. Nixon & Muller (p. 476), 1997. Hardin, 1979
Description
HABIT Perennial, deciduous, phanerophytic, tree, diclinous and monoecious, 25-30 m tall.
STEMS Main stems ascending or erect, round. Bark dark gray to almost black, with large ridges on older specimens, not exfoliating. Branches erect or ascending or horizontal. Twigs brown or gray, fluted-terete, 2-5 mm in diameter, smooth and lenticellate, glabrous or 1st year twigs sparsely beset with long simple and fascicled brown to light brown erect to spreading hairs. Pith white, 5-pointed, continuous, nodal diaphragm absent. Sap translucent. For an anatomical study of the xylem see Tillson & Muller, 1942.
BUDS Terminal and axillary present, clustered at twig apices and scattered along stem. Terminal bud ovoid, blunt; axillary buds 1 per axil, ovoid, blunt. Bud scales brown to light brown, imbricate, with short and unbranched erect or appressed, brown to light brown hairs, sparsely to moderately dense, distributed throughout and especially marginally. Bud scale scars encircling the twig. Leaf scars half-round to crescent-shaped. Vascular bundle scars numerous, scattered.
LEAVES Alternate, simple, (appearing pseudo-opposite or pseudo-whorled at twig apices), crowded toward stem apex or spaced somewhat evenly along and divergent from stem. Stipules lateral, free from the petiole, linear, caducous. Petiole adaxially flattened, 0.4-2 cm long, with long and simple and two-five-armed fasciculate light-brown to brown erect or spreading hairs, sparsely distributed throughout; not glabrescent. Leaf blades: abaxial surface light green (occasionally semi-glaucous), adaxial surface green, oblanceolate or elliptic or obovate, bilaterally symmetric, 5-22 cm long, 4-13 cm wide, chartaceous, pinnately veined; base acute or cuneate; margin regularly crenate to serrate with teeth apices obtuse to sometimes semi-acute, lacking bristle tips; apex acute to obtuse. Abaxial surface minutely papillose and with simple and two-five-armed fasciculate light brown or white hairs, mostly erect, moderately densely distributed throughout; not glabrescent. Adaxial surface glabrous or with simple and with two-five armed fasciculate light brown or white hairs, erect or spreading, sparsely distributed throughout. Minute multi-cellular bulbous glandular hairs also present on both surfaces (Hardin, 1979a)(Thompson & Mohlenbrock, 1979). For an overview of the phenology and the variability and density of hairs due to ecological factors and hybridization see Hardin, 1979b .
FEMALE INFLORESCENCES Coetaneous, spike consisting of a single flower (sometimes 2-3), in axils of current year leaves, subsessile initially, sometimes becoming short-pedunculate in fruit, surrounded by a cupule which is persistent, accrescent, and indurate in fruit (acorn cap). There has been debate over the years regarding the true ontogenetic nature of the cupule. Originally thought to be an involucre of bracts, recent research suggests that the cupule is a complex partial inflorescence derived from stem tissue, see Abbe, 1974; Brett, 1964; Foreman, 1966; MacDonald, 1979; Fey & Endress, 1983. Each cupule subtended by 3 minute, caducous bracteoles.
FEMALE FLOWERS Perianth of one whorl, minute, fragrance absent. Calyx urceolate, of fused sepals. Carpels 3. Locules 3, each containing 2 ovules. Styles 3, each with 1 stigma. Ovary inferior. Placentation axile.
MALE INFLORESCENCES Coetaneous, compound, solitary or fascicled spikes; pendant, catkin-like; in leaf axils of previous year. Rachis moderately covered with brown to white simple and fasciculate hairs; elongating with age, with 1-3 sessile flowers per node, each flower subtended by a small, sessile caducous bracteole.
MALE FLOWERS Perianth of one whorl, 2-2.5 mm in diameter, fragrance absent. Calyx actinomorphic, campanulate, of fused sepals. Sepal lobes 3-8(10), ovate-obovate with brown pilosity, moderately dense to densely distributed throughout. Stamens (4)6-9(12), exserted, surrounding tuft of brown hairs. Anthers glabrous, basifixed, opening along the long axis. Filaments free, 1mm long, straight, glabrous (Rowlee, 1900). For a review of pollen morphology see Solomon, 1983b.
FRUITS Acorn (glans (Spjut, 1994)) (calybium (Kaul, 1985)) subsessile or pedunculate to 2 cm; maturation annual. Acorn ovoid-oblong, 2-3.5 cm long, comprised of 2 parts- a. the crateriform cup (cupule), enclosing about 1/3 of the base of the nut; and b. the nut, 1-seeded by abortion (acorns reported with 2-3 seeds (Coker, 1904) (Smith, 1914). For a hypothesis that the first ovule fertilized suppresses the normal development of the others see Mogensen, 1975. Cupule exterior composed of indurate, imbricate, tightly appressed scales moderately to densely covered with brown to grey tomentum. Nut olive-green to brown, ovoid-oblong, with large light-colored circular cupule scar at base and apiculate at the distal end, glabrous, essentially smooth (minutely laterally striate). One study found a size range of 1.01-10.25 cubic cm with a mean of 3.76 cubic cm and a correlation between smaller acorn size with increasing latitude see Aizen & Woodcock, 1992.
SEEDS Embryo with two large fleshy cotyledons, endosperm lacking. (Young & Young, 1992)
Habitat
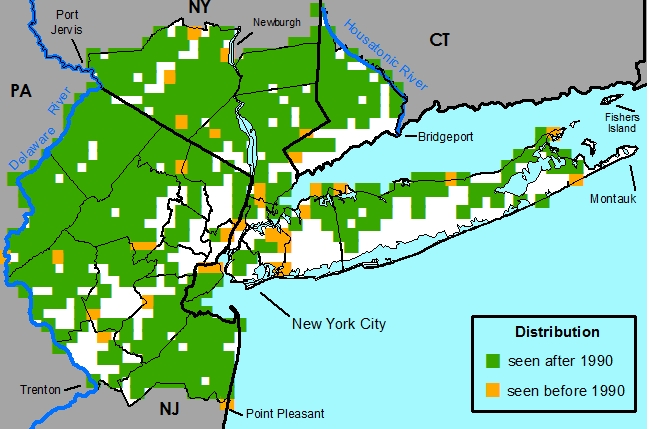
Usually occurring in mesic to dry rocky and sandy substrates in forests, ridges, and barrens.Distribution
Indigenous to the eastern United States.United States -- AL, CT, DE, GA, IL, IN, KY, MA, MD, ME, MI, MS, NC, NH, NJ, NY, OH, PA, RI, SC, TN, VA, VT, WV
New York Metropolitan Region -- Native throughout the metropolitan region.
Rarity Status
Global Heritage Rank -- G5Connecticut -- Not listed
New Jersey -- Not listed
New York -- Not listed
Species Biology
Flowering
May [week 1] - June [week 1] Pollination
Anemophily Fruiting
August [week 1] - October [week 4] Masting, for an investigation on the effects of weather on acorn yields see Sharp & Sprague, 1967. Dispersal
Small predators of acorns facilitate dispersal by dropping undamaged nuts and failing to recover cached nuts. These include Sciurus carolinensis (gray squirrel), Sciurus niger (fox squirrel), Glaucomys volans (Southern Flying Squirrel), Tamias striatus (eastern chipmunk), Peromyscus leucopus (white-footed mouse), Peromyscus maniculatus (deer mouse), Quiscalus quiscula (common grackle) and Cyanocita cristata (blue jay). (Ivan & Swihart, 2000) (Smith, 1972) (Briggs & Smith, 1989) (Wolff, 1996) (Bosema, 1979) (Darley-Hill & Johnson, 1981) (Johnson & Webb, 1989) (Johnson, et al., 1993) (Vaughan, 1991).
In addition, one study found that many predators preferred the basal end of the acorn and consumed only 30-60% of the cotyledon. A chemical analyses of acorns from two species revealed that the concentration of protein-precipitable phenolics (primarily tannins) was 12.5% (Q. phellos) and 84.2% (Q. laevis) higher in the apical portion of the seeds where the embryo is located, suggesting that many acorn consumers consistently eat only a portion of the cotyledon of several species of acorns and thereby permit embryo survival. (Steele, et al., 1993).
Probably included in the diet of the grey fox (Urocyon cinereoargenteus) (Scott, 1955), eastern wild turkeys (Meleagris gallopavo silvestris) (Norman, 2003), and white-tailed deer (Odocoileus virginianus) (Bryant, et al., 1996).
Germination
Acorns of members of the white oak group have little or no dormancy and germinate naturally soon after falling, germination if hypogeal. One study of fall-planted acorns in experimental seedbeds found germination rates of 65-100% across different US provenances (Santamour & Schreiner, 1961).
General rules for collecting and storing acorns: 1. Collect acorns before they lose much water. 2. Ensure acorns are fully hydrated, soak in clean tap water overnight before placing them in storage. 3. Surface dry the acorns just before depositing them in storage to reduce mold growth. 4. Place acorns into cold storage as soon after collection as is possible. (Connor, 2004)
For a propagation protocol for growing bareroot oaks see Hoss, 2004.