Quercus bicolor Willd. - Swamp White Oak
Common Names
Swamp White OakField Identification
Large deciduous tree with alternate, simple, lobed leaves; with pendant catkin-like flowers in early summer followed by acorns on long stalks in the fall.Food uses
Disclaimer: The information provided here is for reference and historical use. We do not recommend nor do we condone the use of this species for food purposes without first consulting a physician.
Native American often used the acorns for a variety of food products, usually by removing the tannins by boiling the nuts or soaking them in lye water. (Moerman, 1998)
Medicinal uses
Disclaimer: The information provided here is for reference and historical use. We do not recommend nor do we condone the use of this species for medicinal purposes without first consulting a physician.
Used by Native Americans to treat a variety of maladies including cholera, broken bones, and tuberculosis. Also used for psychological purposes including compound decoctions for "when wife runs around, takes away loneliness". (Moerman, 1998)
Other uses
Wood prized for cabinet work, flooring, finish carpentry, and barrels for beer, wine, and other alcoholic spirits. (Hill, 1952)
Tannins derived from oaks have been used historically to tan animal hides into leather. (Burrows & Tyrl, 2001)
Oak-mast surveys may help wildlife agencies better understand dynamics of fall harvests and may be useful in harvest management models that attempt to stabilize fall harvest rates of game animals (Norman, 2003).
Poisonous Properties
Oak leaves, buds, bark, and acorns contain tannins which have varying degrees of toxicity in different animals. Although oak foliage and acorns provide valuable food for many wildlife species and even some livestock, oak toxicosis, a urinary and digestive tract disease can occur when some animals are forced to subsist on oaks exclusively for several days. Poisoning is rare in humans due to the large amounts needed to ingest to cause symptoms. (Burrows & Tyrl, 2001)
Nomenclature
Quercus bicolor Willd., Ges. Naturf. Freunde Berlin Neue Schriften 3: 396. 1801.Quercus prinus var. (Q. platanoides) Lam., Encycl. Meth. Bot. 1: 720. 1785.
Quercus alba palustris Marsh., Arbust. Am. 120. 1785.
Quercus prinus (tomentosa) Michx., Hist. Chenes Am. No. 5, t. 9, fig. 2. 1801.
Quercus bicolor var. mollis Nutt., Gen. N. Amer. Pl. 2: 215. 1818.
Quercus prinus var. (Q. bicolor) Spach, Hist. Nat. Veg. Phan. 11: 158. 1842.
Quercus bicolor var. platanoides A. deCan., Prodr. 16,2: 21. 1864.
Quercus bicolor var. angustifolia Dippel, Laubh. 2: 87. 1892.
Quercus bicolor var. lyrata Dippel, Laubh. 2: 87. 1892.
Quercus bicolor var. cuneiformis Dippel, Laubh. 2: 87. 1892.
Quercus discolor var. bicolor Hampton, Ann. Rep. Ohio State For. Bur. 1: 195. 1886.
Quercus platanoides (Lam.) Sudworth, Rep. U.S. Sec. Agric. 1892: 327. 1893.
TYPE: unknown
Description
HABIT Perennial, deciduous (occasionally marcescent), phanerophytic, tree, diclinous and monoecious, 25-30 m tall.
STEMS Main stems ascending or erect, round. Bark light gray to dark gray, scaly to flat-ridged. Branches erect or ascending or horizontal, exfoliating. Twigs brown to gray, fluted-terete, 2-5 mm in diameter, smooth and lenticellate, glabrous or sparsely beset with long and unbranched or fasciculate erect or spreading, brown or light brown hairs on 1st year twigs only. Pith white, 5-pointed, continuous, nodal diaphragm absent. Sap translucent. For an anatomical study of the xylem see Tillson & Muller, 1942;Williams, 1939. For an anatomical study of seedling roots see Carpenter & Guard, 1954.
BUDS Terminal and axillary present, clustered at twig apices and scattered along stem, sessile. Terminal bud ovoid to subglobose, blunt; axillary buds ovoid to subglobose, blunt. Bud scales dark brown or brown, imbricate, glabrous. Bud scale scars bud scale scars encircling the twig. Leaf scars half-round-crescent. Vascular bundle scars numerous and scattered.
LEAVES Alternate, simple, (appearing pseudo-opposite or pseudo-whorled at twig apices), crowded toward stem apex or spaced somewhat evenly along and divergent from stem. Stipules lateral, free from the petiole, linear, caducous. Petiole adaxially flattened, 0.3-1.5 cm long, glabrous or with long and unbranched or fasciculate brown hairs, sparsely to moderately densely distributed throughout. Leaf blades: abaxial surface light green (can appear light gray-whitish due to dense covering of hairs), velvety to touch, adaxial surface green, usually glossy, obovate or elliptic or narrowly elliptic or oblanceolate, bilaterally symmetric, 8-23 cm long, 4-16 cm wide, chartaceous, pinnately veined; base cuneate; margin somewhat regularly lobed 1/4 - 1/3 the distance to the midvein, sinuses acute, lobe apices acute or obtuse, lacking bristle tips (sometimes proximal 1/3 of blade margin entire). Abaxial surface with erect simple hairs, erect 2-8-rayed fasciculate hairs, and appressed 6-16-rayed rotate-stellate hairs, light gray to white or light brown, moderately dense or dense, distributed throughout, and minute multi-cellular bulbous glandular red hairs also present, see Hardin, 1979a; Q. bicolor var. mollis Nutt. has less hair density and appears more greenish (Thompson & Mohlenbrock, 1979). Adaxial surface glabrous or with simple or fasciculate light gray to white hairs or light brown, erect or appressed or spreading, sparsely distributed throughout or distributed along midveins. For an overview of the phenology and the variability and density of hairs due to ecological factors and hybridization see Hardin, 1979b.
FEMALE INFLORESCENCES Coetaneous, spike consisting of a single flower (sometimes 2-3), in axils of current year leaves, subsessile initially, becoming pedunculate in fruit, surrounded by a cupule which is persistent, accrescent, and indurate in fruit (acorn cap). There has been debate over the years regarding the true ontogenetic nature of the cupule. Originally thought to be an involucre of bracts, recent research suggests that the cupule is a complex partial inflorescence derived from stem tissue, see Abbe, 1974;Brett, 1964;Foreman, 1966;MacDonald, 1979;Fey & Endress, 1983. Each cupule subtended by 3 minute, caducous bracteoles.
FEMALE FLOWERS Perianth of one whorl, minute, fragrance absent. Calyx urceolate, of fused sepals. Carpels 3. Locules 3, each containing 2 ovules. Styles 3, each with 1 stigma. Ovary inferior. Placentation axile.
MALE INFLORESCENCES Coetaneous, compound, solitary or fascicled spikes; pendant, catkin-like; in leaf axils of previous year. Rachis moderately covered with brown hairs, elongating with age, with 1-3 sessile flowers per node, each flower subtended by a small, sessile caducous bracteole.
MALE FLOWERS Perianth of one whorl, 2-3 mm in diameter, fragrance absent. Calyx actinomorphic, campanulate, of fused sepals, abaxial and adaxial surfaces brown. Sepal lobes 3-6, broadly oval to ovate with brown pilosity, moderately dense to densely distributed throughout. Stamens (2)3-10(16), exserted, surrounding tuft of brown hairs. Anthers glabrous, basifixed, opening along the long axis. Filaments free, 1mm long, straight, glabrous. For a review of pollen morphology see Solomon, 1983b.
FRUITS Acorn (glans (Spjut, 1994)) (calybium (Kaul, 1985)) pedunculate (peduncle glabrous or with a few light-brown hairs, eglandular) to 7 cm; maturation annual. Acorn ovoid-oblong, 2.5-3 cm long, comprised of 2 parts- a. the crateriform cup (cupule), enclosing about 1/2 (up to 3/4?) of the base of the nut; and b. the nut, 1-seeded by abortion (rarely 2-3 seeded?). For a hypothesis that the first ovule fertilized suppresses the normal development of the others see Mogensen, 1975. Cupule exterior composed of indurate, imbricate, somewhat appressed scales moderately to densely covered with brown to grey tomentum. Nut olive-green to brown, ovoid-oblong, with large light-colored circular cupule scar at base and apiculate at the distal end, with minute short and unbranched or fasciculate brown hairs, sparse or moderately dense, distributed throughout but usually more dense apically, essentially smooth (minutely laterally striate). One study found a size range of .71-6.08 cubic cm with a mean of 2.36 cubic cm and a correlation between smaller acorn size with increasing latitude see Aizen & Woodcock, 1992.
SEEDS Embryo with two large fleshy cotyledons, endosperm lacking. (Young & Young, 1992).
Habitat
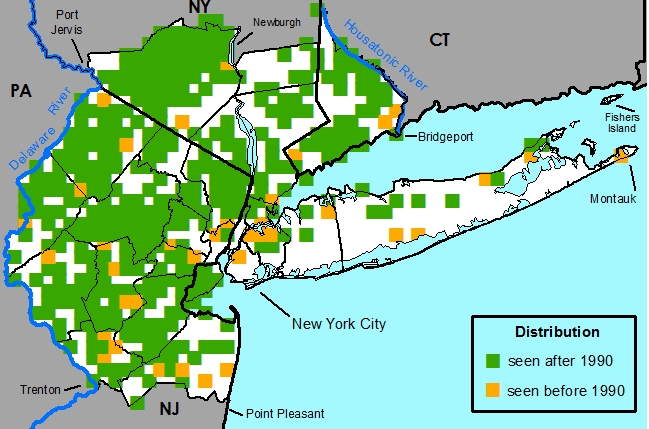
Moist to mesic lowlands, woods, swamps, marshes, and poorly drained upland areas.Distribution
Indigenous to eastern North America.
United States-- AL, CT, DE, IA, IL, IN, KY, MA, MD, ME, MI, MN, MO, NC, NH, NJ, NY, OH, PA, RI, TN, VA, VT, WI, WV
Canada -- ON, QC
New York Metropolitan Region -- Native throughout the metropolitan region.
Rarity Status
Global Heritage Rank -- G5
Connecticut -- Not listed
New Jersey -- Not listed
New York -- Not listed
Species Biology
Flowering April [week 4] - May [week 4]
Pollination Anemophily
Fruiting August [week 3] - October [week 3]
Dispersal Small predators of acorns facilitate dispersal by dropping undamaged nuts and failing to recover cached nuts. These include Sciurus carolinensis (gray squirrel), Sciurus niger (fox squirrel), Glaucomys volans (Southern Flying Squirrel), Tamias striatus (eastern chipmunk), Peromyscus leucopus (white-footed mouse), Peromyscus maniculatus (deer mouse), Quiscalus quiscula (common grackle) and Cyanocita cristata (blue jay). (Ivan & Swihart, 2000) (Smith, 1972) (Briggs & Smith, 1989) (Wolff, 1996) (Bosema, 1979) (Darley-Hill & Johnson, 1981) (Johnson & Webb, 1989) (Johnson, et al., 1993) (Vaughan, 1991).
In addition, one study found that many predators preferred the basal end of the acorn and consumed only 30-60% of the cotyledon. A chemical analyses of acorns from two species revealed that the concentration of protein-precipitable phenolics (primarily tannins) was 12.5% (Q. phellos) and 84.2% (Q. laevis) higher in the apical portion of the seeds where the embryo is located, suggesting that many acorn consumers consistently eat only a portion of the cotyledon of several species of acorns and thereby permit embryo survival. (Steele, et al., 1993).
Probably included in the diet of the grey fox (Urocyon cinereoargenteus) (Scott, 1955), eastern wild turkeys (Meleagris gallopavo silvestris) (Norman, 2003), and white-tailed deer (Odocoileus virginianus) (Bryant, et al., 1996).
Germination (Rink & Williams, 1984) (Young & Young, 1992) (Schopmeyer, 1974) Acorns of members of the white oak group have little or no dormancy and germinate naturally soon after falling, germination is hypogeal. For germination to occur the moisture content of the acorns must not drop below 30-50%. It is usually impractical to store acorns for more than 6 months, however storage in sealed containers or in sand at 32-36dF is probably best. In general, seed of the white oaks lose viability more rapidly in storage over winter than seed from the black oaks. In one study (Q. phellos and Q. laevis), germination experiments revealed equal or greater germination frequencies for partially consumed acorns than for intact acorns. (Steele, et al., 1993).
General rules for collecting and storing acorns: 1. Collect acorns before they lose much water. 2. Ensure acorns are fully hydrated, soak in clean tap water overnight before placing them in storage. 3. Surface dry the acorns just before depositing them in storage to reduce mold growth. 4. Place acorns into cold storage as soon after collection as is possible. (Connor, 2004)
For a propagation protocol for growing bareroot oaks see Hoss, 2004.